When we talk about chemical bonds, we can distinguish between ionic and covalent bonds. Do you know how to identify each type of bond?
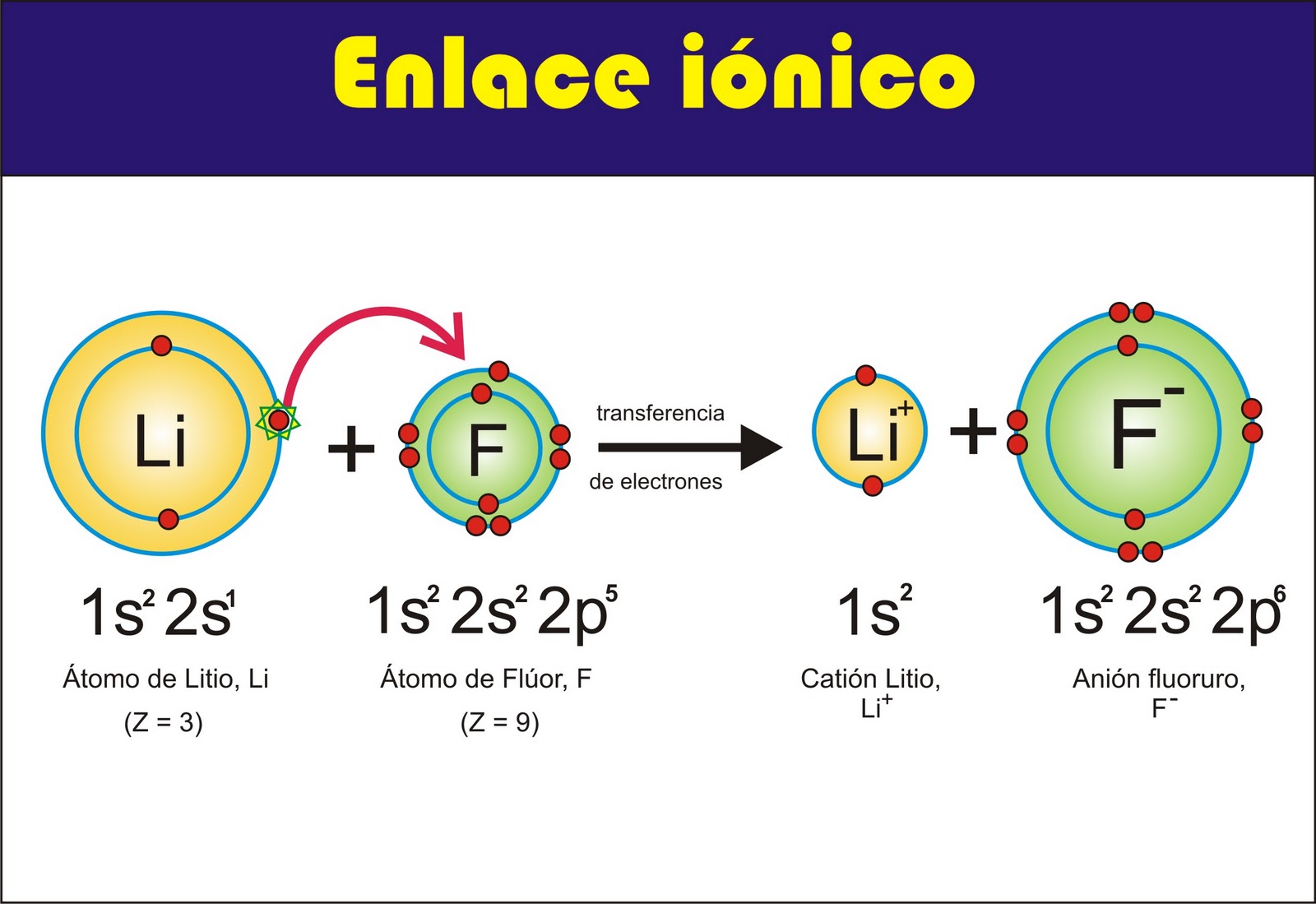
Ionic bonds are formed between a metal and a nonmetal, while covalent bonds are formed between two nonmetals. In an ionic bond, the metal gives up one or more electrons to the nonmetal, resulting in the formation of positively and negatively charged ions. In a covalent bond, the two nonmetals share one or more pairs of electrons.
Here are some examples of ionic and covalent bonds:
- Ionic bond: NaCl (sodium chloride)
- Covalent bond: H2O (water)
Ionic and covalent bonds are both important types of chemical bonds. Ionic bonds are found in many inorganic compounds, such as salts and minerals. Covalent bonds are found in many organic compounds, such as hydrocarbons and proteins.
The type of bond that is formed between two atoms depends on the electronegativity of the atoms. Electronegativity is a measure of how strongly an atom attracts electrons. The more electronegative an atom, the more strongly it attracts electrons. If the difference in electronegativity between two atoms is great, then an ionic bond will form. If the difference in electronegativity between two atoms is small, then a covalent bond will form.
Ejemplos de Ejercicios de Enlaces Inicos y Covalentes
Los enlaces inicos y covalentes son dos tipos importantes de enlaces qumicos que se forman entre tomos. Los enlaces inicos se forman entre un metal y un no metal, mientras que los enlaces covalentes se forman entre dos no metales.
- Formacin: Los enlaces inicos se forman mediante la transferencia de electrones, mientras que los enlaces covalentes se forman mediante el compartimiento de electrones.
- Fuerza: Los enlaces inicos son generalmente ms fuertes que los enlaces covalentes.
- Solubilidad: Los compuestos inicos son generalmente solubles en agua, mientras que los compuestos covalentes no suelen serlo.
- Conductividad: Los compuestos inicos son buenos conductores de la electricidad cuando estn fundidos o disueltos, mientras que los compuestos covalentes son malos conductores.
- Ejemplos: El cloruro de sodio (NaCl) es un ejemplo de un compuesto inico, mientras que el metano (CH4) es un ejemplo de un compuesto covalente.
- Aplicaciones: Los compuestos inicos se utilizan en una amplia variedad de aplicaciones, incluyendo la produccin de sal, fertilizantes y medicamentos. Los compuestos covalentes se utilizan en una amplia variedad de aplicaciones, incluyendo la produccin de plsticos, combustibles y productos farmacuticos.
Los enlaces inicos y covalentes son esenciales para comprender la qumica. Los diferentes tipos de enlaces dan lugar a diferentes propiedades de los compuestos qumicos. Al comprender los enlaces inicos y covalentes, podemos predecir las propiedades de los compuestos qumicos y disear nuevos materiales con propiedades especficas.
Formacin
The formation of chemical bonds is a fundamental concept in chemistry. There are two main types of chemical bonds: ionic bonds and covalent bonds. Ionic bonds are formed between atoms of metals and nonmetals, while covalent bonds are formed between atoms of nonmetals.
- Ionic Bonds
Ionic bonds are formed when one atom transfers one or more electrons to another atom. The atom that loses electrons becomes a positively charged ion, and the atom that gains electrons becomes a negatively charged ion. The oppositely charged ions are attracted to each other by the electrostatic force, forming an ionic bond.
- Covalent Bonds
Covalent bonds are formed when two atoms share one or more pairs of electrons. The shared electrons are attracted to the nuclei of both atoms, forming a covalent bond. Covalent bonds are typically stronger than ionic bonds because the electrons are shared between the atoms.
The type of bond that forms between two atoms depends on the electronegativity of the atoms. Electronegativity is a measure of how strongly an atom attracts electrons. The more electronegative an atom, the more strongly it attracts electrons. If the difference in electronegativity between two atoms is great, then an ionic bond will form. If the difference in electronegativity between two atoms is small, then a covalent bond will form.
Ionic and covalent bonds are essential for understanding the structure and properties of matter. Ionic bonds are found in many inorganic compounds, such as salts and minerals. Covalent bonds are found in many organic compounds, such as hydrocarbons and proteins.
Fuerza
Ionic and covalent bonds are the two main types of chemical bonds. Ionic bonds are formed between atoms of metals and nonmetals, while covalent bonds are formed between atoms of nonmetals. The strength of a chemical bond is determined by the amount of energy required to break the bond. Ionic bonds are generally stronger than covalent bonds because the electrostatic attraction between the oppositely charged ions is stronger than the attraction between the shared electrons in a covalent bond.
- Electronegativity
The electronegativity of an atom is a measure of its ability to attract electrons. The more electronegative an atom, the more strongly it attracts electrons. The difference in electronegativity between two atoms determines the type of bond that will form. If the difference in electronegativity is great, then an ionic bond will form. If the difference in electronegativity is small, then a covalent bond will form.
- Bond Length
The bond length is the distance between the nuclei of the two atoms in a bond. The bond length is inversely proportional to the strength of the bond. The shorter the bond length, the stronger the bond.
- Bond Energy
The bond energy is the amount of energy required to break a bond. The bond energy is directly proportional to the strength of the bond. The greater the bond energy, the stronger the bond.
The strength of chemical bonds is important for understanding the properties of matter. The strength of the bonds between the atoms in a material determines its hardness, melting point, and other physical properties.
Solubilidad
The solubility of ionic and covalent compounds is a fundamental property that affects their behavior in various chemical and biological systems. The difference in solubility between ionic and covalent compounds arises from the nature of the chemical bonds that hold them together.
- Ionic Compounds
Ionic compounds are formed by the electrostatic attraction between positively charged metal ions and negatively charged nonmetal ions. These compounds are generally soluble in water because water molecules are polar, meaning they have a positive end and a negative end. The positive end of the water molecule is attracted to the negative ions in the ionic compound, and the negative end of the water molecule is attracted to the positive ions in the ionic compound. This attraction between the water molecules and the ions in the ionic compound causes the ionic compound to dissolve in water.
- Covalent Compounds
Covalent compounds are formed by the sharing of electrons between nonmetal atoms. These compounds are generally not soluble in water because water molecules are polar, and covalent compounds are nonpolar. Nonpolar molecules do not have a positive end and a negative end, so they are not attracted to water molecules. This lack of attraction between the water molecules and the covalent compound causes the covalent compound to be insoluble in water.
The solubility of ionic and covalent compounds is an important property that affects their use in various applications. For example, ionic compounds are often used in fertilizers because they are soluble in water and can be easily absorbed by plants. Covalent compounds are often used in plastics and other materials that need to be water-resistant.
Conductividad
Conductivity is a measure of a material's ability to conduct electricity. The conductivity of a material depends on the number of free electrons in the material. Free electrons are electrons that are not bound to any particular atom. In metals, there are a large number of free electrons, which is why metals are good conductors of electricity.
Ionic compounds are compounds that are formed by the transfer of electrons from one atom to another. The resulting ions are held together by electrostatic forces. When ionic compounds are dissolved in water, the ions separate and become free to move. This is why ionic compounds are good conductors of electricity when they are dissolved in water.
Covalent compounds are compounds that are formed by the sharing of electrons between atoms. The resulting molecules are held together by covalent bonds. Covalent bonds are stronger than ionic bonds, so covalent compounds are not as good conductors of electricity as ionic compounds.
The conductivity of a compound is an important property that can affect its use in various applications. For example, ionic compounds are often used in batteries because they are good conductors of electricity. Covalent compounds are often used in plastics and other materials that need to be electrical insulators.
The understanding of the conductivity of ionic and covalent compounds is essential for the development of new materials and technologies.
Ejemplos
The examples of ionic and covalent compounds, sodium chloride (NaCl) and methane (CH4), respectively, provide a foundation for understanding the fundamental differences between these two types of chemical bonds. These examples serve as building blocks for further exploration of the properties and behaviors of ionic and covalent compounds.
- Formation and Properties
Ionic compounds, like NaCl, result from the transfer of electrons between metal and nonmetal atoms, leading to the formation of positively charged cations and negatively charged anions. Covalent compounds, exemplified by CH4, involve the sharing of electron pairs between nonmetal atoms. This distinction in bond formation influences various properties, including solubility, conductivity, and melting points. - Solubility
Ionic compounds tend to exhibit high solubility in polar solvents like water due to the strong electrostatic interactions between ions and polar water molecules. Conversely, covalent compounds often display limited solubility in polar solvents because of their nonpolar nature and weaker intermolecular forces. - Conductivity
Ionic compounds, when dissolved in water or melted, dissociate into their constituent ions, enabling the conduction of electricity. Covalent compounds, on the other hand, lack free ions and are generally poor conductors of electricity. - Melting Points
Ionic compounds typically possess higher melting points compared to covalent compounds. The strong electrostatic forces between ions require a significant amount of energy to overcome, resulting in higher melting points. Covalent compounds, with their weaker intermolecular forces, exhibit lower melting points.
These facets collectively contribute to the diverse applications of ionic and covalent compounds. Ionic compounds find use in electrolytes, fertilizers, and various industrial processes, while covalent compounds play crucial roles in organic chemistry, pharmaceuticals, and plastics.
Aplicaciones
The connection between "Aplicaciones: Los compuestos inicos se utilizan en una amplia variedad de aplicaciones, incluyendo la produccin de sal, fertilizantes y medicamentos. Los compuestos covalentes se utilizan en una amplia variedad de aplicaciones, incluyendo la produccin de plsticos, combustibles y productos farmacuticos." and "ejemplos de ejercicios de enlaces ionicos y covalentes" lies in the fact that the different types of chemical bonds lead to different properties of the compounds, which in turn determine their applications.
Ionic compounds, formed by the electrostatic attraction between positively charged metal ions and negatively charged nonmetal ions, are generally soluble in water and good conductors of electricity. These properties make ionic compounds useful for a variety of applications, including:
- Production of salt: Sodium chloride (NaCl), an ionic compound, is the most common type of salt used for food seasoning and preservation.
- Fertilizers: Ionic compounds such as ammonium nitrate (NH4NO3) and potassium chloride (KCl) are widely used as fertilizers to provide essential nutrients for plant growth.
- Medicines: Many ionic compounds are used in medicine, including sodium bicarbonate (NaHCO3) for indigestion and magnesium sulfate (MgSO4) for treating seizures.
Covalent compounds, formed by the sharing of electrons between nonmetal atoms, are generally insoluble in water and poor conductors of electricity. These properties make covalent compounds useful for a variety of applications, including:
- Production of plastics: Covalent compounds such as polyethylene (C2H4)n and polyvinyl chloride (C2H3Cl)n are the building blocks of many plastics, which are used in a wide range of applications from packaging to construction.
- Fuels: Covalent compounds such as methane (CH4) and propane (C3H8) are commonly used as fuels for heating and cooking.
- Pharmaceuticals: Many covalent compounds are used in pharmaceuticals, including aspirin (C9H8O4) for pain relief and ibuprofen (C13H18O2) for inflammation.
The understanding of the connection between the type of chemical bond and the properties of the compound is essential for the development of new materials and technologies. By understanding the fundamental principles of ionic and covalent bonding, scientists and engineers can design and synthesize compounds with specific properties for a wide range of applications.
FAQs on Ionic and Covalent Bonds
This section addresses frequently asked questions (FAQs) on ionic and covalent bonds, providing concise and informative answers to enhance understanding of these fundamental chemical concepts.
Question 1: What is the key difference between ionic and covalent bonds?
Answer: Ionic bonds result from the transfer of electrons between atoms, leading to the formation of oppositely charged ions. In contrast, covalent bonds arise from the sharing of electron pairs between atoms.
Question 2: How does electronegativity influence the type of bond formed?
Answer: Electronegativity, a measure of an atom's attraction for electrons, plays a crucial role. A large difference in electronegativity between atoms favors ionic bond formation, while a small difference promotes covalent bond formation.
Question 3: Are ionic compounds generally soluble in water?
Answer: Yes, ionic compounds tend to dissolve readily in water due to the strong electrostatic forces between ions and polar water molecules.
Question 4: Why are covalent compounds often poor conductors of electricity?
Answer: Covalent compounds lack free ions, which are necessary for the conduction of electricity. The absence of mobile ions limits their ability to conduct electrical current.
Question 5: Provide an example of an ionic compound and its application.
Answer: Sodium chloride (NaCl), common salt, is an ionic compound widely used for food seasoning and preservation.
Question 6: Give an example of a covalent compound and its significance.
Answer: Polyethylene (C2H4)n, a covalent compound, serves as the backbone of many plastics, finding applications in packaging, construction, and various industries.
Summary: Understanding the distinctions between ionic and covalent bonds is essential for comprehending the behavior and properties of chemical compounds. These concepts underpin various fields of science and technology, enabling researchers and scientists to design and develop new materials and applications.
Transition to the next article section: This concludes the FAQs on ionic and covalent bonds. The following section will delve into the applications and significance of these bonds in the realm of chemistry.
Conclusion
Ionic and covalent bonds are the two fundamental types of chemical bonds that govern the formation and properties of compounds. They arise from distinct mechanisms of electron transfer and sharing, leading to unique characteristics in solubility, conductivity, and melting points.
Understanding the nature of these bonds is crucial for comprehending the behavior of matter and the design of new materials. By harnessing the principles of ionic and covalent bonding, scientists and engineers can develop compounds with tailored properties for diverse applications, from energy storage to medicine.
The Essential Guide To Mulch Or Bark Chips: Enhance Your Landscaping
Understanding The Difference Between Call By Value And Call By Reference
When Does The Enchanting Rose Parade Begin?
TIPOS DE ENLACE QUÍMICO

Enlace iónico qué es, cómo funciona

¿Cómo se unen los átomos? Física Química